 Nancy
J. Woolf, Ph.D.
Nancy
J. Woolf, Ph.D.
Laboratory of NanoNeuroscience
|
|

Laboratory of NanoNeuroscience |
My research interests focus upon nanoscale structures in the CNS and the participation of these structures in higher cognition. Particular interests include:
Alzheimer's disease, an aging-related disorder affecting memory and intellectual function, is associated with two types of neuropathology: senile plaques and neurofibrillary tangles. There is also a marked cholinergic deficit in forebrain cholinergic projection neurons. A major impetus underlying my primary research interests is how these pathologies and the cholinergic deficit are related. The fundamental basic science question is how acetylcholine impacts upon the cytoskeleton during higher cognition.
Nanoscale technology and proteomics stand to greatly advance our understanding of cellular functions and operations of intact proteins. Since the human and mouse genomes have been sequenced to near completion, we know that approximately 30,000 proteins are translated. What we still do not fully understand is how most molecules mechanically operate: what controls where and when they interact. These phenomena are particularly important in informational processing. When viewing the individual living cell as a bioinformational device, we need to know: What is the nature of its internal hardware?
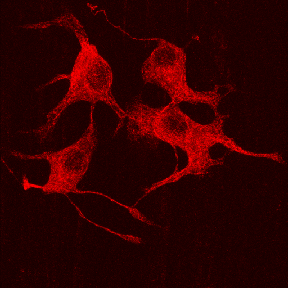
The cytoskeleton is a major component of all living cells. These are thin rod-like filaments that span all regions of the cytoplasm. There are three major types of filaments in neurons: actin-based microfilaments, tubulin-based microtubules (see illustration above), and intermediate filaments called neurofilaments.
Microtubules
are long hollow filaments made of alpha-beta-tubulin dimers. These filaments
have outer diameters measuring ~25 nm and inner diameters of ~15 nm. Tubulin
can account for as much as 10% of the cell’s protein.
Microtubules form through the polymerization of alpha-beta-tubulin dimers in a GTP-dependent process. Strands or protofilaments are often formed first. Microtubules consist of 13 such protofilaments. Each mm of microtubule length consists of 1650 heterodimers.
Associated proteins. Proteins that bind microtubules perform a number of functions in cells. Examples include motor proteins, such as kinesin, which move macromolecular structures to precise destinations in the cytoplasm and structural proteins in neurons (MAPs) that organize proteins near to synapses.
MAPs attach to specific sites on microtubules and thereby affect computational processing executed by microtubules. Neurons contain a number of MAPs, prominent among which are MAP-2 and tau. MAP-2 is present in brain as several isoforms: MAP-2a and MAP-2b are the high molecular weight components (280 kD and 270 kD, respectively); MAP-2c is smaller (70 kD). MAP-2b is presently early and late during postnatal development, whereas MAP-2a appears only late (appearing between days 10 - 20 in rat). MAP-2c is only found in appreciable levels early in development for most brain areas; however, it plays a role in learning in the adult hippocampus (next section). Unless specified otherwise, MAP-2 generally refers to MAP2ab.
MAP-2 and tubulin proteolysis alters the structural organization within the dendrite during learning and memory consolidation (Woolf, 1996; 1998; Woolf et al., 1999). There are good reasons to believe that this reorganization of MAP-2 is relevant to information processing. Messenger RNA for MAP-2 is localized to dendrites, along with several other mRNA species, many or all of which may play a role in activity-dependent synapse modification related to learning and memory (Kiebler & DesGroseillers, Neuron, 25:19, 2000; Steward & Schuman, Ann. Rev. Neurosci. 24:299, 2001). MAP-2 appears to serve as an anchoring protein for the cAMP-dependent kinase: PKA (Harada et al., J Cell Biol., 158:541, 2002). Kinesin protein, a microtubule motor, contributes to neuronal events required for learning and memory; knockout mice are impaired on memory tasks (Wong et al., PNAS, 99:14500, 2002).
Site-specific phosphorylation of MAP-2 potently affects its ability to bind to microtubules. Cholinergic muscarinic activation acting through phosphoinositide-specific phospholipase C (PI-PLC), which then turns on protein kinase C (PKC) and Ca2+/calmodulin dependent kinase II (C/CMK), leads to increased phosphorylation of the MAP-2 protein. MAP-2 has over 40 sites of phosphorylation (see Johnson and Jope, J. Neurosci. Res. 33:505, 1994; Sanchez et al., Prog. Neurobiol. 61:133, 2000). Other monoamine neuromodulators (i.e., serotonin, dopamine and norepinephrine) and metabotropic glutamate receptors modulate MAP-2 phosphorylation, in part by activating PI-PLC, and also through either activation or inhibition of adenylyl cyclase. Nonetheless, acetylcholine is around 10 times more concentrated in the mammalian cerebral cortex than other monoamines. Hence among the neuromodulators, cholinergic muscarinic effects are in a position to produce the greatest increase in MAP-2 phosphorylation.
Acetylcholine has been suggested to play a prominent role in the mediation of memory (Woolf et al., 2001) and conscious activity in brain (Woolf, 1997; Perry et al., TINS, 22:273, 1999; Woolf and Hameroff, 2001). Acetylcholine muscarinic receptors affecting the phosphorylation of MAP-2 has implications for quantum computing. Quantum computing in brain microtubules may be tuned by MAP-2 according to Penrose-Hameroff Orch OR (Penrose, 1994; Penrose and Hameroff, 1995). Quantum computing in microtubules, and classical computing involving electrophysiological events around the membrane, may work together (see figure below).
Microtubule-associated protein-2 and Memory
|
|
MAP-2 immunohistochemistry highlights the cells that participate saliently in learning and memory consolidation (Woolf, 1996; 1998; Woolf et al., 1999). MAP-2 is highly concentrated in the large layer 5 pyramidal cells of the cerebral cortex and of hippocampus. There are also some small MAP-2 rich cells throughout layers 2 - 6. Considering the large cell body volume along with numerous MAP-2 filled dendrites belonging to the large layer 5 pyramidal cells, it is clear that the large layer 5 pyramidal cells contain the vast majority of MAP-2 protein. Nearly the exact same population of cells contains high levels of muscarinic receptor immunoreactivity and other cytoskeletal proteins (Woolf, 1993). |
|
|
|
Control Tone paired Tone upaired |
|
MAP-2 shows evidence of increased degradation in CA1 and CA2, i.e., increased immunostaining (shown above) and increased presence of breakdown products (not shown) with memory consolidation of a context (Woolf et al., 1999). Rats placed in a novel training chamber and then given tone followed by shock will exhibit behavioral evidence of having acquired a fear of the chamber. The fear-related behavior is freezing, a crouching, immobilized posture that is atypical for rats, which normally actively explore a novel chamber, sniff, or groom themselves. MAP-2 changes occur in the temporal cortex when shock onset immediately follows tone offset, but not when tone and shock are spaced apart by 5 minutes (Woolf et al., 1994). However, with fear conditioning to the chamber (i.e., the context in which the training takes place), both tone paired and unpaired with shock produces MAP-2 changes in CA1 and CA2 of hippocampus. |
| Central cholinergic pathways are ideally suited to regulate global functions that rely upon the cerebral cortex; such functions include attention, arousal, motivation, memory and consciousness (Woolf, 1991; Woolf, 1996). The basal forebrain contains two groups of cholinergic neurons: (1) the medial septal group (medial septal nucleus and vertical diagonal band: ms and vdb) that project cholinergic axons to the hippocampus and parahippocampal gyrus and (2) the nucleus basalis group (nucleus basalis, substantia innominata and horizontal diagonal band: bas, si, hdb) that project cholinergic axons to all parts of the neocortex, parts of limbic cortex and to the amygdala. The cholinergic pontomesencephalon neurons (laterodorsal tegmental and pedunculopontine tegmental nuclei: ldt and ppt) project onto hindbrain, thalamus, hypothalamus and basal forebrain. |
|
|
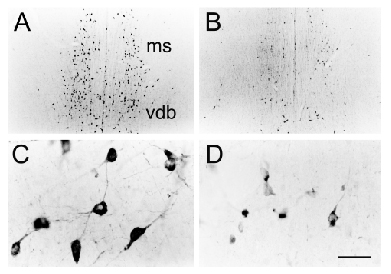 |
Cholinergic neurons participate in learning and memory. This
is illustrated by increased soma cross-sectional areas of nucleus basalis
cells containing mRNA for choline acetyltransferase (ChAT) along with
increased level of ChAT mRNA (Oh et
al., 1996).
Since cholinergic neurons depend on NGF and high affinity TrkA receptors, one can block cholinergic function with oligonucleotides to TrkA (Woolf et al., 2001). This leads to reduced somal size (see left panel). |
| (Right panel) Reductions in somal cross-sectional areas as visualized by ChAT immunohistochemistry and vesicular acetylcholine transporter (VAChT) immunohistochemistry following TrkA oligonucleotide knockdown of these proteins were found to be significantly correlated with the behavioral measures of context memory (i.e., freezing to the chamber). | 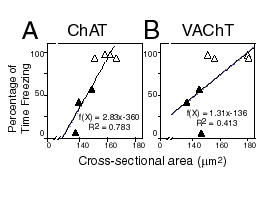 |
|
|
| After Woolf and Hameroff 2001: A Quantum Approach to Visual Consciousness. Abbreviations: Ca2+/calmodulin-dependent kinase II (C/CMK); classical computing (CC); dopamine receptors (DA); GABA receptors (GABA-B; GABA-A); ionotropic glutamate receptors (AMPA/Kainate); metabotropic glutamate receptors (mGlu); microtubule-associated protein-2 (MAP-2); muscarinic acetylcholine receptor (mACh); norepinephrine receptors (NE); orchestrated objective reduction (Orch OR); phosphoinositide-specific phospholipase C (PI-PLC); protein kinase A (PKA); protein kinase C (PKC); quantum computing (QC); serotonin receptors (5-HT). |
| Psychology 10 | Psychology 15 | Psychology 119P |
| Office: | Address: | Phone, FAX: | Email: |
| 8633 Franz Hall |
Dept. of Psychology University of California Los Angeles, CA 90095-1563
|
(310) 206-7874
(310) 206-5895
|
woolf@psych.ucla.edu |
Photo Gallery
| 2000 Trip to Göteborg, Sweden | 1999 Trip to Alaska |
Biography
|
B.S.-- Psychobiology-- UCLA Dept. of Psychology--1978 |
Ph.D.--Neuroscience--UCLA School of Medicine--1983 |
1. Woolf,
N.J. and Butcher, L.L. Cholinergic neurons in the caudate-putamen complex proper
are intrinsically-organized: a combined Evans Blue and acetylcholinesterase
analysis. Brain Research Bulletin, 1981, 7,
487-507.
2. Bigl, V.,
Woolf, N.J., and Butcher, L.L. Cholinergic projections from the basal forebrain
to frontal, parietal, temporal, occipital, and cingulate cortices: a combined
fluorescent tracer and acetylcholinesterase analysis. Brain
Research Bulletin, 1982, 8,
727-749.
3. Butcher,
L.L. and Woolf, N.J. Cholinergic and serotonergic systems in the brain and
spinal cord: anatomic organization, role in intercellular communication
processes, and interactive mechanisms. Progress
in Brain Research, 1982, 55,
3-40.
4. Butcher,
L.L. and Woolf, N.J. Monoaminergic-cholinergic relationships and the chemical
communication matrix of the neostriatum and substantia nigra. Brain
Research Bulletin, 1982, 9,
475-492.
5. Woolf,
N.J. and Butcher, L.L. Cholinergic projections to the basolateral amygdala: a
combined Evans Blue and acetylcholinesterase analysis.
Brain Research Bulletin, 1982,
8, 751-763.
6. Woolf,
N.J., Eckenstein, F. and Butcher, L.L. Cholinergic projections from the basal
forebrain to the frontal cortex: A combined fluorescent tracer and
immunohistochemical analysis. Neuroscience
Letters, 1983, 40, 93-98.
7. Butcher,
L.L. and Woolf, N.J. Histochemical distribution of acetylcholinesterase in the
central nervous system: Clues to the localization of cholinergic neurons. In: Handbook of Chemical Neuroanatomy, Volume 3: Classical Transmitters and
Transmitter Receptors in the CNS, Part II. (Eds.: A. Björklund, T. Hökfelt,
and M.J. Kuhar). Amsterdam: Elsevier Biomedical Press, 1984, pp. 1-50.
8. Woolf,
N.J. Eckenstein, F., and Butcher, L.L. Cholinergic systems in the rat brain: I.
Projections to the limbic telencephalon. Brain
Research Bulletin, 1984, 13,
751-784.
9. Woolf,
N.J. and Butcher, L.L. Cholinergic systems in the rat brain: II. Projections to
the interpeduncular nucleus. Brain
Research Bulletin, 1985, 14,
63-83.
10. Butcher,
L.L. and Woolf, N.J. Central cholinergic systems: synopsis of anatomy and
overview of physiology and pathology. In: The
Biological Substrates of Alzheimer's Disease (Eds.:
A.B. Scheibel and A.F. Wechsler) New York: Academic Press, 1986, pp. 73-86.
11. Butcher,
L.L. and Woolf, N.J. Cholinergic systems in the brain and spinal cord: anatomic
organization and overview of functions. In: Alzheimer's
and Parkinson's Disease: Strategies for Research and Development (Eds.: A.
Fisher, I. Hanin and C. Lachman) New York: Plenum Press, 1986, pp. 5-16.
12. Butcher,
L.L. and Woolf, N.J. Cholinergic systems in the central nervous system:
retrospection, anatomic distribution, and functions. In: Dynamics
of Cholinergic Function (Ed.:
I. Hanin). New York: Plenum Press, 1986, pp. 1-10.
13. Woolf,
N.J. and Butcher, L.L. Cholinergic systems in the rat brain: III. Projections
from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia,
and basal forebrain. Brain Research Bulletin, 1986,
16, 603-637.
14. Woolf,
N.J., Hernit, M.C., and Butcher , L.L. Cholinergic and non-cholinergic
projections from the rat basal forebrain revealed by combined choline
acetyltransferase and Phaseolus vulgaris immunohistochemistry. Neuroscience
Letters, 1986, 66, 281-286.
15. Butcher,
L.L. and Woolf, N.J. Cholinergic neuronal regeneration can be modified by growth
factor. In: Cellular and Molecular Basis of Cholinergic Function (Eds.: M.J.
Dowdall and J.N. Hawthorne) Chichester, U.K.: Ellis Horwood, 1987, pp. 395-402.
16.
Harrison, J.B., Buchwald, J.S., Kaga, K., Woolf, N.J., and Butcher, L.L. Cat
"P300" disappears after septal lesions. EEG
and Clinical Neurophysiology, 1988,
69, 55-64.
17. Talbot,
K., Woolf, N.J., and Butcher, L.L. The feline islands of Calleja complex I.
Cytoarchitectural organization and comparative anatomy. Journal
of Comparative Neurology, 1988, 275,
553-579.
18. Talbot,
K., Woolf, N.J., and Butcher, L.L. The feline islands of Calleja complex
II. Cholinergic and cholinesterasic features. Journal
of Comparative Neurology, 1988, 275,
580-603.
19. Butcher,
L.L. and Woolf, N.J. Dysdifferentiation initiates and growth processes
exacerbate the pathologic cascade in Alzheimer's disease. Neurobiology
of Aging, 1989, 10, 557-570.
20. Butcher,
L.L. and Woolf, N.J. Authors' response to commentaries. Neurobiology
of Aging, 1989, 10, 588-590.
21. Gould,
E., Woolf, N.J., and Butcher, L.L. Cholinergic projections to the substantia
nigra from the pedunculopontine and laterodorsal tegmental nuclei. Neuroscience,
1989, 28: 611-623.
22. Woolf,
N.J. and Butcher, L.L. Cholinergic systems in the rat brain IV. Descending
projections from the pontomesencephalon. Brain
Research Bulletin, 1989, 23,
519-540.
23. Woolf,
N.J., Gould, E., and Butcher, L.L. Nerve growth factor receptor is associated
with cholinergic neurons of the basal forebrain but not the pontomesencephalon. Neuroscience,
1989, 30, 143-152.
24. Woolf,
N.J., Jacobs, R.W., and Butcher, L.L. The pontomesencephalotegmental cholinergic
system does not degenerate in Alzheimer's disease. Neuroscience
Letters, 1989, 96,
277-282.
25.
Harrison, J., Woolf, N.J., and Buchwald, J.
Cholinergic neurons of the pontomesencephalon: I. Role in the generation
of the cat "P1". Brain
Research, 1990, 520, 43-54.
26. Woolf,
N.J. and Butcher, L.L. Dysdifferentiation of structurally plastic neurons
initiates the pathologic cascade of Alzheimer's disease: toward a unifying
hypothesis. In: Cholinergic Systems
(Eds.: M. Steriade and D. Biesold). New York: Oxford University Press, 1990, pp.
387-438).
27. Woolf,
N.J., Harrison, J., and Buchwald, J. Cholinergic
neurons of the pontomesencephalon: II. Ascending anatomical connections.
Brain Research, 1990, 520,
55-72.
28. Gould,
E., Woolf, N.J., and Butcher, L.L. The postnatal development of cholinergic
systems in the rat I. Forebrain. Brain
Research Bulletin, 1991, 27,
767-789.
29. Oh,
J.D., Butcher, L.L., and Woolf N.J. Thyroid
hormone modulates the development of cholinergic terminal fields in the rat
forebrain. Developmental Brain Research, 1991, 59, 133-142.
30. Woolf,
N.J. Cholinergic systems in
mammalian brain and spinal cord. Progress
in Neurobiology, 1991, 37,
475-524.
31. Woolf,
N.J. and Butcher, L.L. The cholinergic basal forebrain as a cognitive machine.
In: Functions of the Basal Forebrain Cholinergic System (Ed.: R.T.
Richardson), 1991, pp. 347-380.
32. Butcher,
L.L., Oh, J.D., Woolf, N.J., Edwards, R.H., and Roghani, A. Organization of
central cholinergic neurons revealed by in
situ hybridization histochemistry and choline-o-acetyltransferase
immunocytochemistry. Neurochemistry
International, 1992, 21, 429-445.
33. Kaga,
K., Harrison, J., Butcher, L.L.,
Woolf, N.J., and Buchwald, J. Cat
"P300" and cholinergic septo-hippocampal neurons: Depth recordings,
lesions, and choline acetyltransferase immunohistochemistry.
Neuroscience Research, 1992, 13,
53-71.
34. Oh,
J.D., Woolf, N.J., Roghani, A., Edwards, R.H., and Butcher, L.L.
Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization
of choline acetyltransferase mRNA, Neuroscience,
1992, 47, 807-822.
35. Butcher,
L.L., Oh, J.D., Woolf, N.J. Cholinergic
neurons identified by in situ hybridization histochemistry.
Progress in Brain Research,
1993, 98, 1-8.
36. Farris,
T.W., Woolf, N.J., Oh, J.D., and Butcher, L.L. Reestablishment of laminar
patterns of cortical acetylcholinesterase activity following axotomy of the
medial cholinergic pathway in the adult rat.
Experimental Neurology, 1993, 121,
77-92.
37. Woolf,
N.J. Cholinoceptive cells in rat cerebral cortex:
somatodendritic immunoreactivity for muscarinic receptor and cytoskeletal
proteins. Journal
of Chemical Neuroanatomy, 1993, 6, 375-390.
38. Woolf,
N.J., Young, S.L., Johnson, G.V.W. & Fanselow, M.S. Pavlovian fear
conditioning alters microtubule-associated protein-2. NeuroReport,
1994, 5, 1045-1048.
39. Farris,
T.W., Oh, J.D., Butcher, L.L. and Woolf, N.J. Trophic-factor modulation of
cortical acetylcholinesterase reappearance following transection of the medial
cholinergic pathway in adult rat. Experimental
Neurology, 1995, 131, 180-192.
40. Oh,
J.D., Edwards, R.H. and Woolf, N.J. Choline acetyltransferase mRNA plasticity
with Pavlovian conditioning to tone. Experimental
Neurology, 1996, 140,
95-99.
41. Woolf,
N.J. Book Review: Journey to the Centers of the Mind: Toward a Science of
Consciousness, by Susan A. Greenfield. Neuroscience,
1996, 72, 1156.
42. Woolf,
N.J. Global and serial neurons form
a hierarchically-arranged interface proposed to underlie learning and cognition.
Neuroscience, 1996, 74,
625-651.
43. Woolf,
N.J. The critical role of
cholinergic basal forebrain neurons in morphological change and memory encoding:
a hypothesis. Neurobiology of Learning and Memory, 1996, 66, 258-266.
44. Woolf,
N.J. and Oh, J.D. Thyroid hormone effects on the postnatal development of
microtubule-associated protein-2 (MAP-2): comparisons with MAP-1 and MAP-5. In: Advances
in Neuroendocrinology - Thyroid
Hormone and Brain Maturation. (Ed. C.E. Henrich), 1997, pp. 31-38.
45. Woolf,
N.J. A possible role for
cholinergic neurons of the basal forebrain and pontomesencephalon in
consciousness. Consciousness
and Cognition, 1997, 6, 574-596.
46. Woolf,
N.J. A structural basis for memory
storage in mammals. Progress in Neurobiology, 1998,
55, 59-77.
47. Woolf, N.J., Zinnerman, M.D. and Johnson G.V.W. Hippocampal microtubule-associated protein-2 alterations with contextual memory. Brain Research, 1999, 821: 241-249.
48. Woolf, N.J. Cholinergic correlates of consciousness: from mind to
molecules. Trends in Neurosciences, 1999, 22: 540-541.
49. Woolf, N.J. Dendritic encoding: An alternative to temporal synaptic
coding of conscious experience. Consciousness
and Cognition, 1999, 8: 574-596.
50.
Woolf, N.J. Review: An Anatomy of Thought: The Origin and Machinery of Mind, by
Ian Glynn. American Scientist, Sept.-Oct., 2000, p. 459.
51. Woolf, N.J., Milov, A., Schweitzer, E.S. and Roghani, A. Elevation of nerve growth factor and antisense knockdown of TrkA receptor during contextual memory consolidation . Journal of Neuroscience, 2001, 21, 1047-1055.
52. Woolf, N.J. and Hameroff, S.R. A quantum approach to visual consciousness. Trends in Cognitive Sciences, 2001, 5, 472-478.
53.
Woolf, N.J. Cholinergic anatomy and consciousness: potential for a novel type of
signal transduction. In: The
Neurochemistry of Consciousness: Neurotransmitter in Mind.
(Eds. E. Perry, H. Ashton & A. Young), 2001.
Copyright notices
Selected articles on this webpage are available for viewing and printing single copies for personal research and study only. Permission granted by Elsevier Science and Academic Press.